Unlock the Secrets of Food and Drug Regulations: Explore the Code of Federal Regulations Title 21 Parts 99

In the ever-changing landscape of food and drug regulations, staying informed is crucial. The Code of Federal Regulations (CFR) Title 21 Parts 99 provides a comprehensive roadmap, guiding professionals through the intricate web of regulations governing the production, distribution, and use of food and drugs in the United States.
This detailed explanation will delve into the significance, structure, and key features of CFR Title 21 Parts 99, empowering you with the knowledge to navigate the regulatory landscape with confidence.
4.6 out of 5
| Language | : | English |
| File size | : | 1634 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 333 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
The Importance of CFR Title 21 Parts 99
CFR Title 21 Parts 99 establishes the regulatory framework for medical devices, ensuring their safety, effectiveness, and quality. By adhering to these regulations, manufacturers, distributors, and healthcare providers can protect the public from harmful or ineffective medical devices.
The regulations cover a wide range of topics, including:
- Device classification and premarket approval requirements
- Good manufacturing practices for medical devices
- Medical device reporting and surveillance
- Enforcement and compliance
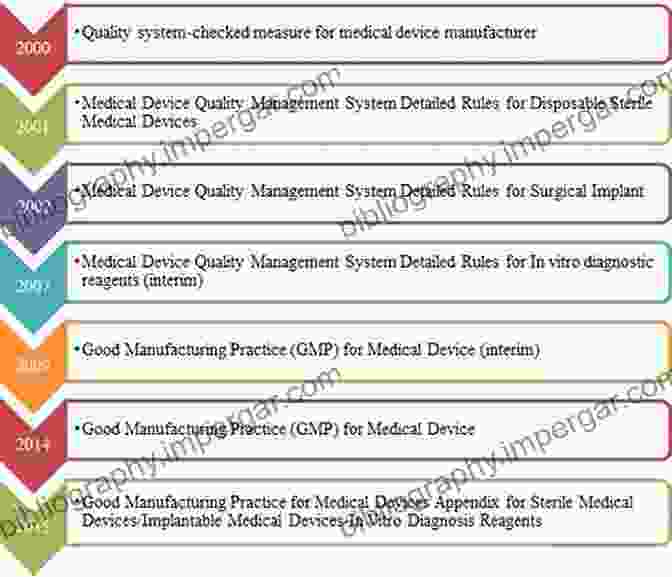
Structure of CFR Title 21 Parts 99
CFR Title 21 Parts 99 is organized into several subparts, each addressing a specific aspect of medical device regulations. Here's an overview:
- Subpart A: General Provisions - Defines the scope, purpose, and definitions used throughout the regulations.
- Subpart B: Classification and Reclassification of Devices - Establishes the classification process for medical devices and provides criteria for reclassification.
- Subpart C: Premarket Notification - Outlines the premarket notification requirements for Class II and Class III medical devices.
- Subpart D: Premarket Approval - Specifies the premarket approval requirements for Class III medical devices that pose significant risks.
- Subpart E: Production and Quality System Regulation - Sets forth the good manufacturing practices and quality system requirements for medical device manufacturers.
- Subpart F: Postmarket Surveillance - Establishes requirements for medical device reporting, monitoring, and surveillance.
- Subpart G: Enforcement and Inspections - Outlines the enforcement and inspection procedures for ensuring compliance with the regulations.
Key Features of CFR Title 21 Parts 99
Here are some notable features that make CFR Title 21 Parts 99 essential:
- Comprehensive Coverage: The regulations provide a comprehensive overview of the regulatory requirements for medical devices, covering all aspects from classification to enforcement.
- Legal Authority: These regulations are legally binding and enforceable by the Food and Drug Administration (FDA),ensuring compliance within the medical device industry.
- Regular Updates: The CFR is updated regularly to reflect changes in technology and scientific knowledge, ensuring that the regulations remain current.
- Clear Guidance: The regulations are written in clear and concise language, providing straightforward guidance for compliance.
- Organized Structure: The subparts and sections within CFR Title 21 Parts 99 are well-organized, making it easy to navigate and locate the relevant information.
CFR Title 21 Parts 99 serves as an indispensable resource for anyone involved in the medical device industry. By understanding the regulations outlined in this document, manufacturers, distributors, healthcare providers, and consumers can ensure the safety, effectiveness, and quality of medical devices in the United States.
For those seeking in-depth knowledge and practical guidance on CFR Title 21 Parts 99, the Budget Edition 2024 offers a comprehensive and up-to-date resource.
Alt Attributes
*  *
*  *
*  *
*  *
* 
4.6 out of 5
| Language | : | English |
| File size | : | 1634 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 333 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Franz Brentano
Franz Brentano Frank Walker
Frank Walker Roberto Sirvent
Roberto Sirvent Sarah Bartholomeusz
Sarah Bartholomeusz J Hillis Miller
J Hillis Miller Ted Goleman
Ted Goleman Kendra L
Kendra L F Michael Higginbotham
F Michael Higginbotham Gabriela Manzoni
Gabriela Manzoni Katherine Knight
Katherine Knight Jana Marx
Jana Marx Gary Marshall
Gary Marshall Gary F Stasko
Gary F Stasko Heather J Paper
Heather J Paper Gabrielle Bendiner Viani
Gabrielle Bendiner Viani Paco Ignacio Taibo Ii
Paco Ignacio Taibo Ii M A Hayat
M A Hayat Tim R Swartz
Tim R Swartz Fergus M Bordewich
Fergus M Bordewich Roger C Paulson
Roger C Paulson
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Neal WardFrom the Ivory Tower to the Assembly Floor: Unpacking the Politics of Food in...
Neal WardFrom the Ivory Tower to the Assembly Floor: Unpacking the Politics of Food in...
 Benji PowellThe Native American Struggle for Self-Determination and Inclusion: A Journey...
Benji PowellThe Native American Struggle for Self-Determination and Inclusion: A Journey...
 William ShakespeareMothers, Midwives, and the Production Line: A Riveting Account of Birth in...
William ShakespeareMothers, Midwives, and the Production Line: A Riveting Account of Birth in... Neal WardFollow ·8k
Neal WardFollow ·8k Allan JamesFollow ·3.9k
Allan JamesFollow ·3.9k Samuel BeckettFollow ·18k
Samuel BeckettFollow ·18k Donald WardFollow ·12k
Donald WardFollow ·12k Thomas HardyFollow ·3.8k
Thomas HardyFollow ·3.8k Terry PratchettFollow ·12.9k
Terry PratchettFollow ·12.9k Orson Scott CardFollow ·2.7k
Orson Scott CardFollow ·2.7k Jacob FosterFollow ·5.1k
Jacob FosterFollow ·5.1k

 Alexander Blair
Alexander BlairBecoming Sports Agent Masters At Work: The Ultimate Guide
What is a Sports...

 Xavier Bell
Xavier BellUnveiling the Enchanting World of Upper Bohemia: A Review...
A Captivating...

 Chris Coleman
Chris ColemanUnveiling the Secrets: Extreme Rapid Weight Loss Hypnosis...
In the relentless pursuit of a slimmer,...
4.6 out of 5
| Language | : | English |
| File size | : | 1634 KB |
| Text-to-Speech | : | Enabled |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 333 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |